Abstract
BRAF genomic alterations are the most common oncogenic drivers in pediatric low-grade glioma (pLGG). Arm 1 (n = 77) of the ongoing phase 2 FIREFLY-1 (PNOC026) trial investigated the efficacy of the oral, selective, central nervous system–penetrant, type II RAF inhibitor tovorafenib (420 mg m−2 once weekly; 600 mg maximum) in patients with BRAF-altered, relapsed/refractory pLGG. Arm 2 (n = 60) is an extension cohort, which provided treatment access for patients with RAF-altered pLGG after arm 1 closure. Based on independent review, according to Response Assessment in Neuro-Oncology High-Grade Glioma (RANO-HGG) criteria, the overall response rate (ORR) of 67% met the arm 1 prespecified primary endpoint; median duration of response (DOR) was 16.6 months; and median time to response (TTR) was 3.0 months (secondary endpoints). Other select arm 1 secondary endpoints included ORR, DOR and TTR as assessed by Response Assessment in Pediatric Neuro-Oncology Low-Grade Glioma (RAPNO) criteria and safety (assessed in all treated patients and the primary endpoint for arm 2, n = 137). The ORR according to RAPNO criteria (including minor responses) was 51%; median DOR was 13.8 months; and median TTR was 5.3 months. The most common treatment-related adverse events (TRAEs) were hair color changes (76%), elevated creatine phosphokinase (56%) and anemia (49%). Grade ≥3 TRAEs occurred in 42% of patients. Nine (7%) patients had TRAEs leading to discontinuation of tovorafenib. These data indicate that tovorafenib could be an effective therapy for BRAF-altered, relapsed/refractory pLGG. ClinicalTrials.gov registration: NCT04775485.
Main
Pediatric low-grade glioma (pLGG) is the most common childhood central nervous system (CNS) tumor, representing approximately 30% of pediatric brain tumors1. Although considered indolent and potentially curable in certain locations by complete surgical resection2, tumors located in critical areas of the brain not amenable to complete resection may induce functional deficits and/or have an aggressive biology. In such cases, therapies are required that may lead to significant morbidity, with disease progression potentially causing further functional deficits. Given the chronic nature of pLGG, children often require several lines of treatment in their first two decades of life2,3. Treatment goals include regression or stabilization (at a minimum) of disease and neurologic deficits; avoidance of potential acute and long-term toxicities (especially those limiting cognitive functions); and the ability to participate in age-appropriate activities. Frontline systemic therapy currently consists of chemotherapy, which can achieve overall response rates (ORRs) of around 30% and can provide durable responses in some children2,4. However, frequent clinic visits are required; an indwelling catheter is often necessary; and tumors often progress or relapse, necessitating additional treatment4. Moreover, in patients receiving chemotherapy, more than 75% may experience grade 3/4 hematologic toxicity; approximately 20% may experience peripheral neuropathy; and, for patients diagnosed in infancy, the number of chemotherapy regimens may be associated with an increased risk of neurodevelopmental disruption4,5. Although MEK inhibitors may represent an emerging standard of care (SOC) in the United States for patients with relapsed/refractory disease, these daily medications require fasting before and after dosing, which can be particularly challenging in younger children. Moreover, many patients will progress during or after therapy with these agents. There is currently no global uniform consensus on first-line or second-line/further-line SOC in this setting for most patients. Approved therapies that demonstrate treatment response and balance quality of life, with minimal risk of long-term toxicities, are urgently needed for patients with pLGG.
Most pLGGs are driven by genomic alterations affecting components of the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway, which regulates cell proliferation and survival6,7. They are often somatic activating alterations of BRAF, including KIAA1549::BRAF gene fusions or BRAF V600E point mutations8,9. The frequency of BRAF alterations varies across pLGG subtypes, with KIAA1549::BRAF fusions present in 30–40% of tumors overall and in 70–80% of pilocytic astrocytomas, the most common subtype9,10,11,12.
A randomized phase 2 trial, which investigated the type I RAF inhibitor dabrafenib plus the MEK inhibitor trametinib versus vincristine and carboplatin as first-line therapy in patients with pLGG with a BRAF V600 mutation, showed the superiority of dabrafenib plus trametinib over vincristine–carboplatin in this setting13. The US Food and Drug Administration (FDA) recently approved dabrafenib plus trametinib for first-line treatment of patients 1 year of age and older with a pLGG harboring a BRAF V600E mutation who require systemic therapy14,15,16. However, type I BRAF inhibitors—either alone or in combination with a MEK inhibitor—are not indicated for the treatment of patients with tumors harboring BRAF fusions. Type I RAF inhibitors are ineffective in targeting the encoded RAF kinase dimers17 and may cause paradoxical upregulation of MAPK pathway signaling, in BRAF wild-type tumors or tumors harboring BRAF fusions, promoting accelerated tumor growth18,19. For patients with tumors harboring BRAF fusions, or for tumors that progress on or after dabrafenib and/or trametinib therapy, treatment options are limited. Although MEK inhibitor monotherapy has demonstrated early evidence of efficacy in clinical trials in children with progressive pLGG20,21,22,23, it is not approved for use in this setting. Phase 3 trials in the frontline setting are ongoing24.
Tovorafenib (previously known as DAY101, TAK-580, MLN2480 or BIIB024) is an investigational, oral, selective, CNS-penetrant type II RAF inhibitor in clinical development for the treatment of patients with tumors harboring an activating RAF alteration. Studies in murine models have shown that it has potent activity against both oncogenic BRAF fusions, which signal as RAS-independent dimers, and BRAF V600 mutations, which signal as RAS-independent monomers. In contrast to type I BRAF inhibitors, tovorafenib did not induce paradoxical activation of the MAPK pathway in these models25. In an adult phase 1 trial, tovorafenib showed single-agent activity in patients with BRAF-mutated melanoma naive to RAF and MEK inhibitors26. Additionally, anti-tumor activity was reported in a child with a recurrent spindle cell sarcoma harboring a novel SNX8::BRAF fusion and in three adult patients with melanomas harboring different BRAF fusions27,28. The phase 1b PNOC014 (NCT03429803) clinical trial evaluated the safety and tolerability of tovorafenib monotherapy across a dose range up to 530 mg m−2 per week, providing early evidence of anti-tumor activity in patients with BRAF-altered relapsed/refractory pLGG, and supported the selected phase 2 dose of 420 mg m−2 (maximum dose 600 mg) administered orally once per week29.
The phase 2 FIREFLY-1 (PNOC026; NCT04775485) trial objective was to evaluate the safety and efficacy of tovorafenib monotherapy in children, adolescents and young adults with RAF-altered, relapsed/refractory pLGG or advanced solid tumors. Here we report efficacy outcomes for arm 1 and safety outcomes for arms 1 and 2, which enrolled patients 6 months to 25 years of age with pLGG harboring a known activating BRAF (arm 1; registrational) or RAF (arm 2; extension) alteration and is the basis for a US New Drug Application. The primary endpoint of overall response rate (ORR) was assessed per independent radiology review committee (IRC) according to Response Assessment in Neuro-Oncology High-Grade Glioma (RANO-HGG) criteria30, as these were considered the only validated assessment criteria at the time of trial initiation. The Response Assessment in Pediatric Neuro-Oncology Low-Grade Glioma (RAPNO) criteria had been recently published, so efficacy assessments by RAPNO criteria were included as secondary endpoints due to their relevance for this patient population31. Efficacy by RANO-LGG criteria was added as a post hoc exploratory endpoint per regulatory authority request and was prespecified in the updated statistical analysis plan before initiation of the current analysis32. Assessment of changes in quality of life and health utilities measures were exploratory objectives. This report presents the efficacy analysis from the evaluable population in arm 1 (enrolled patients who received ≥1 dose of tovorafenib and met the prespecified efficacy analysis criteria per IRC for the relevant radiological assessment criteria) and safety from arms 1 and 2 (safety analysis set) as of a 5 June 2023 datacut.
Results
Endpoints
The primary endpoint in arm 1 was the ORR as assessed by the IRC according to RANO-HGG criteria. Secondary endpoints for arm 1 included ORR by RAPNO criteria per IRC; clinical benefit rate (CBR), progression-free survival (PFS), duration of response (DOR) and time to response (TTR), per IRC by RANO-HGG and RAPNO criteria; and safety and tolerability (primary endpoint in arm 2), assessed by the type, frequency and severity of adverse events (AEs) and laboratory abnormalities. Additional planned secondary endpoints not reported in this manuscript are described in detail in the full trial protocol (confidential information redacted) in the Supplementary Information and include pharmacokinetics, the effect of tovorafenib on the corrected QT interval by Fredericia (QTcF) and other electrocardiogram (ECG) parameters, change in visual acuity and concordance of molecular profiling approaches. The characteristics of the three different radiological response assessment criteria used in the assessment of efficacy in the trial are summarized in Extended Data Table 1.
Patients and disposition
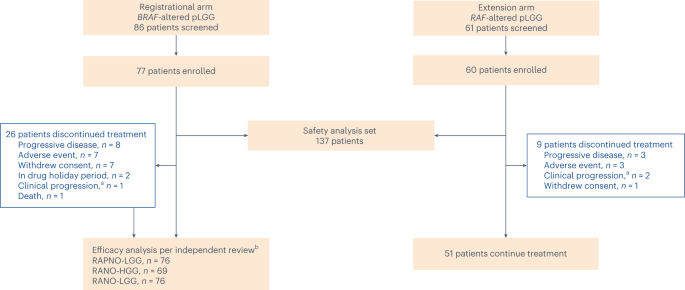
Between 22 April 2021 and 26 January 2023, 137 patients were enrolled to arms 1 and 2 and received tovorafenib—77 in arm 1 and 60 in arm 2. Seven (9%) patients in arm 1 and 21 (35%) patients in arm 2 received tovorafenib as a liquid formulation; all other patients received the tablet formulation. As of the datacut, 102 patients remained on treatment (Fig. 1). The most common reasons for treatment discontinuation were progressive disease (PD) and AEs. Patient demographics and baseline characteristics are summarized in Table 1 and were similar between arms. In all 137 patients, the median age was 9 years (range, 1–24); most patients were White (58%); and most patients had astrocytic tumors (93%). The most common tumor locations were optic pathway (50%) and deep midline structures (15%). Seventy-four percent of patients had a tumor harboring a KIAA1549::BRAF fusion; 10% had a chromosomal rearrangement involving BRAF (as detected by fluorescence in situ hybridization or in situ hybridization and presumed to represent a KIAA1549::BRAF or other BRAF fusion); and 16% had a BRAF V600E mutation. Patients had received a median of three lines of prior therapy (range, 1–10), with 61% having received a prior MEK and/or BRAF inhibitor.
This report presents efficacy data from the evaluable population (enrolled patients who received at least one dose of tovorafenib and met the prespecified efficacy analysis criteria per each radiological assessment method according to the IRC) in arm 1 (registrational) and the safety data from arms 1 and 2 (safety analysis set) as of a 5 June 2023 datacut. Patients were enrolled in arm 2 (extension) after arm 1 had fully accrued and closed for enrollment. aNot radiologically confirmed. bOnly patients with measurable disease at baseline per independent review were included.
The IRC deemed 69 patients in arm 1 who received ≥1 dose of tovorafenib to have measurable disease at baseline per RANO-HGG criteria. Seventy-six patients in arm 1 were deemed evaluable for efficacy by the IRC per RAPNO and RANO-LGG criteria, which use T2-weighted imaging for response assessment. Safety was analyzed in all patients in arms 1 and 2 who received ≥1 dose of tovorafenib (n = 137).
Patients in arm 1 had received a median of 18 treatment cycles (range, 1–26), with median treatment compliance of 100% (range, 93–100) (treatment compliance (%) was the total actual dose (mg) / total expected dose (mg) × 100%). The median duration of treatment (DOT) was 15.8 months (range, 0.7–23.7), with 66% (n = 51) continuing treatment. Two patients had completed 26 treatment cycles and opted to enter a drug holiday period. Neither patient had subsequent imaging after initiation of the drug holiday period. All other patients (except the ones who discontinued drug early) were still on treatment as of the 5 June 2023 datacut. Patients in arm 2 had received a median of 11 treatment cycles (range, 2–15), with median treatment compliance of 100% (range, 83–100). The median DOT was 9.7 months (range, 1.2–13.3), with 85% (n = 51) continuing treatment. There was no decline from baseline in Karnofsky or Lansky performance status scores during treatment with tovorafenib.
RANO-HGG criteria (primary and secondary endpoints)
The ORR per RANO-HGG criteria (primary endpoint) in patients with response-evaluable disease was 67% (95% confidence interval (CI): 54–78), including 12 (17%) patients with a complete response (CR) and 34 (49%) patients with a partial response (PR). Eighteen (26%) patients had a best overall response (BOR) of stable disease (SD), giving a CBR of 93% (Table 2). The ORR was 69% (95% CI: 56–81) in patients with tumors harboring BRAF fusions and 50% (95% CI: 19–81) in patients with tumors harboring BRAF V600E mutations. The ORR was 71% (95% CI: 54–84) in the 59% of evaluable patients who had received prior MAPK inhibitor (MAPKi) therapy and 61% (95% CI: 41–78) in those who had not.
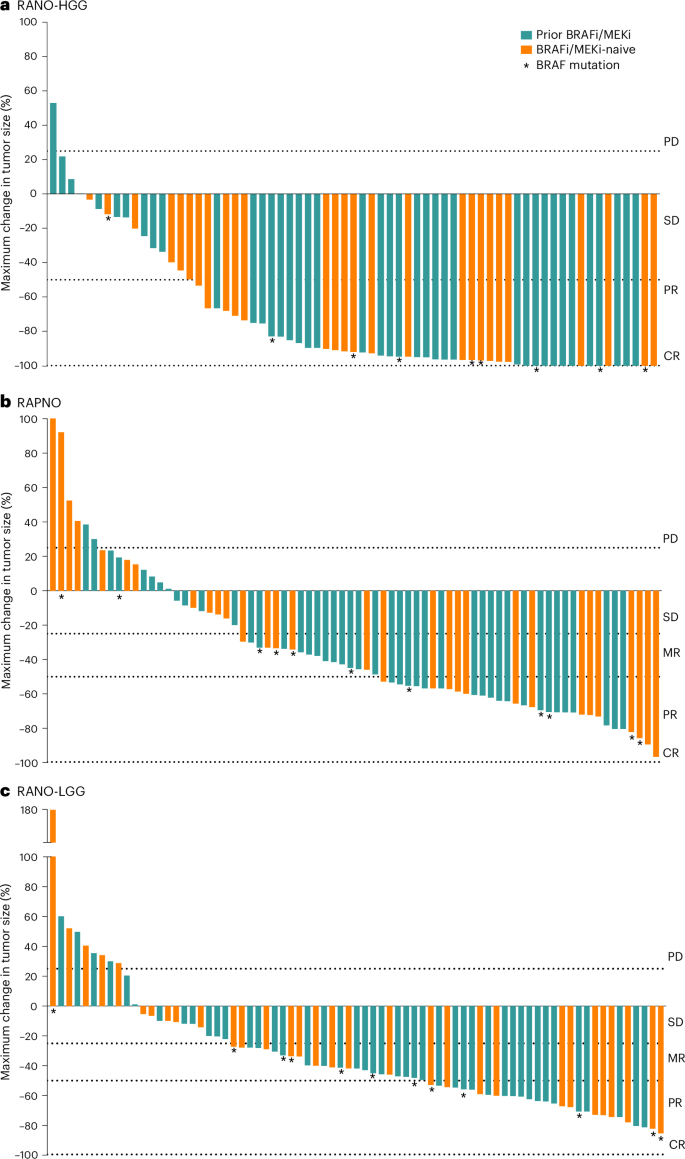
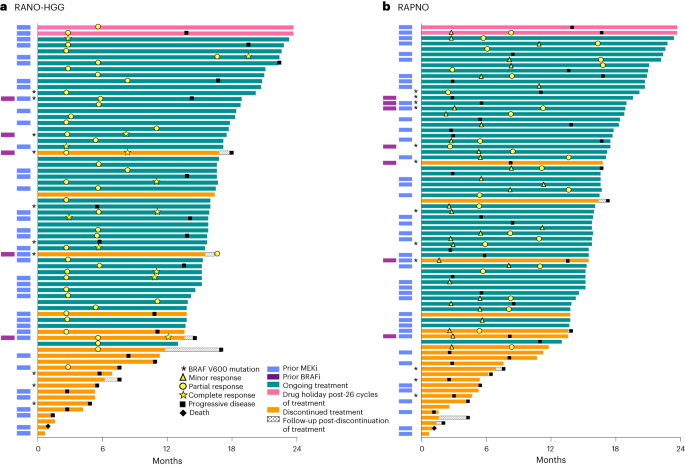
Figure 2a shows the best tumor response from baseline, and Fig. 3a shows DOT and timing of response. In 13% (n = 9) of patients, an initial PR was followed by a CR with continued treatment. The median time to initial response (PR or CR) was 3.0 months (range, 2.6–16.6), and the median DOR was 16.6 months (95% CI: 11.6–not reached (NR)). The median PFS was 19.4 months (95% CI: 16.9–NR). Of the patients who progressed while on therapy per RANO-HGG criteria and continued to receive tovorafenib, 10 had at least one assessment from a scheduled visit after PD. Tumor kinetics for these patients are shown in Extended Data Fig. 1a, with six having tumor shrinkage close to CR.
Per RANO-HGG (a), RAPNO (b) and RANO-LGG (c) criteria. Two patients are not shown in the waterfall plots. One patient died due to PD (not tovorafenib related) before the first tumor assessment, and one patient with missing T1 gadolinium-enhanced imaging at baseline was deemed not evaluable. The dashed lines indicate the range of growth/shrinkage of target lesions to be considered as one of the requirements for PD, SD, MR, PR or CR. BRAFi, BRAF inhibitor.
Per RANO-HGG (a) and RAPNO (b) criteria. In patients with response, symbols indicate the start of response (MR, PR or CR). If initial responses improved with continued treatment (from MR to confirmed PR or from PR to confirmed CR), both the timepoint of the initial response and the timepoint that the response initially improved are marked accordingly. BRAFi, BRAF inhibitor.
RAPNO criteria (secondary endpoints)
The ORR per RAPNO criteria was 51% (95% CI: 40–63), including 28 (37%; 95% CI: 26–49) patients with a PR (≥50% reduction from baseline by sum of the product of the perpendicular diameters (SPPD)) and 11 (14%; 95% CI: 8–24) patients with a minor response (MR; 25–49% reduction from baseline by SPPD). Twenty-three (30%) patients had a BOR of SD, resulting in a CBR of 82%. The ORR was similar in patients with tumors harboring BRAF fusions (52%; 95% CI: 39–64) and BRAF V600E mutations (50%; 95% CI: 21–79) and in patients who had received prior MAPKi therapy (49%; 95% CI: 34–64) and those who had not (55%; 95% CI: 36–73) (Table 2).
Figure 2b shows the best tumor response from baseline, with most tumors showing some degree of shrinkage. Figure 3b shows DOT and timing of response. In 29% (n = 22) of patients, an initial MR was followed by a confirmed PR with continued treatment. The median time to initial response (MR or PR) was 5.3 months (range, 1.6–11.2) overall; 2.8 months (range, 1.6–3.0) in patients with tumors harboring BRAF V600E mutations; and 5.5 months (range, 2.3–11.2) in patients with tumors harboring BRAF fusions. The median DOR was 13.8 months (95% CI: 11.3–NR), and the median PFS of the 76 patients was 13.8 months (95% CI: 8.3–16.9).
Thirty-one patients initially had PD according to RAPNO criteria per IRC evaluation but continued tovorafenib treatment due to the absence of PD by RANO-HGG criteria per investigator, consistent with the trial design and primary endpoint assessment. All but two patients had no symptoms or signs of clinical progression. Plots of tumor size (SPPD) over time in several of these patients showed subsequent tumor shrinkage after the initial assessment of PD (Extended Data Fig. 1b).
RANO-LGG criteria (post hoc exploratory endpoints)
The ORR per RANO-LGG criteria was 53% (95% CI: 41–64), including 20 (26%; 95% CI: 17–38) patients with a PR and 20 (26%; 95% CI: 17–38) with an MR. Twenty-three (30%) patients had a BOR of SD, giving a CBR of 83%. ORR was similar in patients with tumors harboring BRAF fusions and BRAF V600E mutations and in patients previously treated with a MAPKi or not (Table 2).
Figure 2c shows the best tumor response from baseline, again with most tumors showing some decrease in size. Extended Data Fig. 2 shows the DOT and timing of response. In 18% (n = 14) of patients, an initial MR was followed by a confirmed PR with continued treatment. The median time to initial response (MR or PR) was 5.5 months (range, 1.6–11.3) overall; 2.9 months (range, 1.6–11.3) in patients with tumors harboring BRAF V600E mutations; and 5.5 months (range, 2.3–11.0) in patients with tumors harboring BRAF fusions. The median DOR was 14.4 months (95% CI: 11.0–NR), and the median PFS was 13.9 months (95% CI: 11.1–19.1).
Similar to the RAPNO analysis, there was a subset of patients who initially had PD according to RANO-LGG criteria per IRC evaluation but who continued on treatment due to the absence of PD according to RANO-HGG criteria. Plots of tumor size (SPPD) over time in several of these patients showed a pattern of subsequent tumor shrinkage after the initial PD assessment (Extended Data Fig. 1c).
Response to tovorafenib in patients with PD while on a MAPKi as their most recent prior therapy (post hoc analysis)
A post hoc analysis was completed using all three radiological criteria to assess responses to tovorafenib in patients who received a MAPKi as their most recent line of therapy and discontinued due to progression. For RANO-HGG criteria, 33% (n = 15) of patients who had progressed on a MAPKi as their most recent prior therapy had a CR or a PR to tovorafenib; similar trends were observed for RAPNO and RANO-LGG crieria, which include MRs: 33% (n = 13) had a PR or an MR (31% (n = 12) with PR), and 30% (n = 12) had a PR or an MR (20% (n = 8) with PR), respectively (Extended Data Table 2).
Safety
Among 137 patients (arms 1 and 2), 118 (86%) had been treated for at least 6 months and 67 (49%) for at least 1 year. All patients experienced at least one treatment-emergent adverse event (TEAE). The most common TEAEs at any grade occurring in ≥20% of patients were hair color changes (76%), anemia (59%) and elevated creatine phosphokinase (CPK) (58%) (Table 3). Grade ≥3 TEAEs occurred in 63% of patients, the most common being elevated CPK (12%), anemia (11%) and maculopapular rash (8%). The most common treatment-related adverse events (TRAEs) of any grade generally followed a similar trend to the most common TEAEs. (A full listing of TRAEs of any grade and grade ≥3 occurring in ≥1 patient can be found in Supplementary Table 1). Grade ≥3 TRAEs occurred in 42% of patients and followed a similar trend as the most common grade ≥3 TEAEs, although pyrexia was less often assessed as a related event. Two patients had grade 5/fatal TEAEs, neither of which was assessed as treatment related (one patient in arm 1 with disease progression and one patient in arm 2 with a disseminated leptomeningeal mixed-glial-neuronal tumor who experienced tumor hemorrhage 21 d after their final dose of tovorafenib (discontinued due to disease progression)).
A decrease in growth velocity was observed in patients treated with tovorafenib. At cycle 13 (n = 74), the median decrease in height z-score was 0.7, or less than 1 s.d. Eighty percent of these patients remained within 1 s.d. from baseline height z-score. The remaining patients had a decrease from baseline height z-score of between 1 s.d. and 2 s.d. At the time of this report, on-treatment bone age results were available for 11 patients. Nine of these 11 patients were reported as within normal limits for age; one patient was read as delayed; and one patient with a bone age advanced by 3.5 years before the start of treatment had an on-treatment bone age that was reported as advanced by 2.5 years. So far, there has been no evidence of bone age advancement or premature closure of growth plates. In patients with available height data off-treatment, growth velocity was recovering.
Serious TEAEs were reported for 45% of patients, the most common being pyrexia (most cases were confounded by intercurrent infectious disease), seizure and vomiting (each 5%). Serious TRAEs occurred in 15% of patients, the most common being tumor hemorrhage (3%), decreased appetite, hyponatremia and vomiting (each 2%). Two of four patients with serious tumor hemorrhage TRAEs had a history of intratumoral hemorrhage before initiating tovorafenib, and, in all four, the serious events of tumor hemorrhage resolved.
Nine (7%) patients had TRAEs leading to tovorafenib discontinuation, the most common being tumor hemorrhage (three patients) and decrease in growth velocity (two patients). TRAEs leading to dose reduction were seen in 33 (24%) patients, the most common being maculopapular rash (4%) and decreased appetite (3%). TRAEs leading to dose interruption occurred in 50 (37%) patients, the most common being maculopapular rash (9%), vomiting, fatigue, increased alanine aminotransaminase (ALT) and elevated CPK (each 4%). The median duration of dose interruption due to any TEAE was 14 d, or two doses.
Discussion
In this international, multicenter, single-arm phase 2 trial, tovorafenib monotherapy resulted in clinically meaningful, rapid and durable tumor responses in children and young adults with BRAF-altered relapsed/refractory pLGG. The clinical activity of tovorafenib is particularly noteworthy given that patients had received a median of three prior lines of systemic therapy, and more than half had previously received RAF and/or MEK inhibitors. Tumor responses were demonstrated across the three response assessment criteria (RANO-HGG, RAPNO and RANO-LGG), BRAF alteration type (mutation versus fusion) and prior MAPKi use, including patients who progressed on a MAPKi as their most recent prior therapy. There was a consistent pattern of improvement of response over time on both T1-weighted and T2-weighted magnetic resonance imaging (MRI) sequences.
This trial was designed with IRC-assessed ORR as the primary endpoint, evaluated according to RANO-HGG criteria. These criteria assess tumor response based primarily on T1-weighted, contrast-enhanced imaging. At the time the original protocol was initiated, the largest trial in pLGG with published efficacy results in the relapsed/refractory population primarily used T1-weighted, contrast enhancement–based, centrally reviewed assessment criteria to evaluate the treatment effect of vinblastine33. According to RANO-HGG criteria, the FIREFLY-1 trial met its primary endpoint by rejecting the null hypothesis ORR of 21% observed for single-agent vinblastine in this setting34.
For patients with pLGG, decrease in contrast-enhancing tumor may not represent all aspects of anti-tumor activity. The RAPNO working group recommendations highlighted the lack of standard response criteria in clinical trials of pLGG as well as the biologic difference between pediatric and adult gliomas31. RAPNO criteria focus on T2-weighted fluid-attenuated inversion recovery (FLAIR) imaging for assessing response in pLGG rather than changes in contrast-enhancing disease. This is beneficial as pLGGs have clinical and biological features distinct from adult LGGs, and assessment of the contrast-enhancing portion of the tumor may not be the best indicator of response in this population31,35. RAPNO criteria take into account changes in tumor-associated cysts; include an MR category (tumor reduction of 25% to <50%); and recommend including visual outcomes in response assessment (optic pathway and hypothalamic pLGGs), given their clinical importance in pLGG. Therefore, assessment of tumor response by IRC according to RAPNO and RANO-LGG criteria (both of which assess tumor response based primarily on non-enhancing disease by T2/FLAIR) were included in this trial as secondary and post hoc exploratory endpoints, respectively (Extended Data Table 1 summarizes the characteristics of the three different response assessment criteria). Sustained decreases in tumor size were observed in most patients and represent clinically meaningful changes by T2/FLAIR-based assessment criteria. As in the current trial, a similar pattern of response with a greater magnitude of decrease for contrast enhancement assessments compared to T2/FLAIR assessments in patients with relapsed/refractory pLGG was previously reported in a phase 1 trial of the MEK inhibitor selumetinib36. ORRs and CBRs for RAPNO and RANO-LGG criteria were very similar in arm 1. Although in limited numbers of patients, TTR in patients with tumors harboring BRAF V600E mutations appeared to be shorter by both assessment criteria (2.8 months and 2.9 months, respectively) compared to patients with tumors harboring BRAF fusions (5.5 months and 5.5 months).
Although responses to tovorafenib generally occurred early, the kinetic analysis of tumor size over time per RAPNO and RANO-LGG criteria revealed that some patients who remained on treatment after an initial assessment of radiographic PD subsequently had marked tumor shrinkage, suggestive of a delayed response to treatment. This type of response pattern (that is, tumor flare or pseudoprogression) was previously observed in patients treated with immune checkpoint inhibitors, where some patients experience an initial increase in tumor size, followed later by an objective response37,38. It has been suggested that such effects may be related to a transient immune cell infiltration of the tumor, leading to an initial increase in apparent tumor burden37,39. Immune cells, especially microglia, may comprise 40% of all cells in pilocytic astrocytomas and can account for differences in RNA expression profiles between tumor locations and subtypes40. The possibility of a late response to tovorafenib after an apparent initial increase in tumor size highlights the challenge of efficacy evaluation in this patient population using established response assessment criteria, and it raises the possibility that some patients may benefit from tovorafenib treatment until radiographic progression is confirmed by a second MRI scan. Moving forward, follow-up imaging 8–12 weeks after an initial PD assessment will be suggested for patients receiving tovorafenib with early radiographic progression, in the absence of clinical evidence of progression.
By using both T1-weighted contrast-enhanced (RANO-HGG) and T2/FLAIR-based (RAPNO and RANO-LGG) approaches, the current trial assessed the impact of tovorafenib on different aspects of pLGG tumor biology and response. Despite the unique challenges of ascertaining an optimal, single set of response assessment criteria for this heterogeneous disease, confirmed responses to tovorafenib over time were observed across three different neuro-oncology response assessment criteria. To our knowledge, this is the first trial to report outcomes for these three criteria for a large, uniformly treated patient group.
The main limitation of the current trial is that it is a single-arm clinical trial. However, this design was considered both sufficient and necessary due to the lack of a SOC for most patients with relapsed/refractory pLGG. In addition, there was a lack of diversity in relation to ethnicity/race of the patient population. The efficacy results of tovorafenib in FIREFLY-1 are similar to those in earlier non-registrational studies investigating MAPK inhibitors in this setting. The phase 2 trial of the MEKi selumetinib20 demonstrated a sustained PR in nine of 25 patients (36%, excluding MRs) with BRAF-altered pLGG as assessed by T2/FLAIR imaging, with a median time to progression of 22.9 months and a median follow-up of 26.9 months. Other reports of MEKi monotherapy in this patient population demonstrated anti-tumor activity to varying degrees21,22,23,41. The over 50% ORR reported for tovorafenib using RAPNO and RANO-LGG criteria in the current trial is particularly noteworthy given that over half of patients had previously received MAPKi therapy.
The safety and tolerability profile of tovorafenib monotherapy in children and young adults with pLGG was encouraging, with TRAEs being predominantly grade 1 or 2 and only nine (7%) of 137 patients discontinuing due to a TRAE. The most common TRAEs of any grade, excluding laboratory abnormalities, were hair color changes, fatigue, maculopapular rash, dry skin, acneiform dermatitis, pruritus and paronychia. Although grade 1 and 2 laboratory abnormalities were commonly reported as AEs, most were not associated with any clinical symptoms or need for clinical intervention or change in therapy. The most common grade ≥3 TRAEs included elevated CPK, anemia and maculopapular rash. Other common grade ≥3 TEAEs (not assessed as related to tovorafenib), including seizures, were consistent with effects of the underlying disease.
Overall, the AEs were consistent with the tovorafenib phase 1 trial in adults dosed once weekly and similar to other targeted agents used as pLGG therapy, including MAPK inhibitors, with some notable exceptions26. Hair color changes, which have been reported in clinical studies of pediatric patients with pLGG treated with other MAPK inhibitors, occurred more often with tovorafenib42. However, pyrexia, diarrhea and weight gain—common adverse reactions for BRAFi/MEKi combination therapy—were less often reported as tovorafenib related and did not significantly disrupt treatment. No signs of ocular toxicity, adverse impact on cardiac function or abnormal weight gain were observed in children treated with tovorafenib, unlike those observed with MEK inhibitors43,44. Although skin rashes were common in children treated with tovorafenib, no patients experienced life-threatening skin reactions, and development of squamous cell carcinomas and keratoacanthomas were not observed.
There were no on-trial treatment-related deaths, and TRAEs requiring discontinuation were infrequent. Intratumoral hemorrhage was reported in 15 patients and led to discontinuation of therapy in three patients. However, half (eight of 15) of the patients with intratumoral hemorrhage were asymptomatic, with areas of tumor hemorrhage identified on routine trial MRI only. Three of the seven patients with symptomatic tumor hemorrhage had tumor bleeds assessed by the investigator as consistent with the natural history of their underlying tumor and not related to tovorafenib. Of the four patients in which the symptomatic hemorrhage was considered by the investigator to be possibly related to tovorafenib, two patients had a history of tumor hemorrhage before starting the study; the third patient had a disseminated tumor with leptomeningeal disease; and the fourth patient had bone marrow failure secondary to prior treatment with multiple alkylating agents that was diagnosed before the onset of tumor hemorrhage. Although the incidence of intratumoral hemorrhage in this patient population is not well described, case reports suggest that the tumor hemorrhage risk across pLGG tumor types with heterogenous histology, morphology and prior interventions may be underappreciated45,46,47,48.
Decreases in growth velocity were observed in children treated with tovorafenib. Among patients with complete endocrinology assessments reported, radiographic studies of the wrist supported conservation of growth potential with no advancement of bone age or evidence of premature fusion of growth plates. Furthermore, patients with available data after discontinuation of tovorafenib in the setting of FIREFLY-1 and prior studies show various degrees of recovery of growth velocity, including achievement of catch-up growth. A more detailed analysis of growth during and after treatment with tovorafenib is planned. Notably, children with cancer, and with midline CNS tumors such as pLGG, commonly develop endocrine abnormalities that may impact growth trajectories and the likelihood of achieving genetic potential for height. In one report, nearly half of children with hypothalamic/chiasmatic gliomas developed at least one endocrine disorder, most commonly a growth hormone deficiency49. Normative data for growth in this patient population are lacking, and future studies of targeted therapies in pLGG should include baseline and longitudinal assessments of endocrine function and monitoring of growth velocity during therapy. Long-term follow-up of FIREFLY-1 patients after cessation of treatment is ongoing to assess the impact of transient reduction in growth velocity on final adult height.
Collectively, these results show that tovorafenib monotherapy was generally well tolerated and demonstrated encouraging evidence of clinically meaningful, rapid and durable clinical activity in children and young adults with BRAF-altered pLGG. Tovorafenib may, consequently, offer an important treatment option for BRAF-altered, relapsed/refractory pLGG, as the observed safety profile compares favorably to currently available therapies for pLGG with a positive benefit–risk ratio. Notably, the availability of a liquid formulation, a weekly dosing regimen and lack of food effect allow for better adherence to the prescribed treatment regimen. Management of common adverse reactions was achieved in most patients, with only brief dose interruptions. These data provide a strong rationale for the ongoing phase 3 LOGGIC/FIREFLY-2 (NCT05566795) trial of tovorafenib monotherapy versus current SOC chemotherapy in children and young adults with pLGG requiring primary systemic treatment.
Methods
Trial design
FIREFLY-1 (PNOC026; NCT04775485) is an ongoing, phase 2, multicenter, open-label study evaluating tovorafenib monotherapy in children, adolescents and young adults with RAF-altered pLGGs or advanced solid tumors who have received at least one prior systemic therapy. The trial consists of three treatment arms, with patients enrolled from 32 centers in 11 countries (Supplementary Table 2). Arm 1 enrolled patients with relapsed or refractory pLGG harboring an activating BRAF alteration, including BRAF V600 mutations and KIAA1549::BRAF fusions; arm 2 (pLGG expansion cohort) enrolled patients with relapsed or refractory pLGG harboring an activating RAF alteration; and arm 3 is enrolling patients with advanced solid tumors harboring an activating RAF fusion. RAF alterations were identified through molecular assays as routinely performed as part of SOC diagnostic testing at Clinical Laboratory Improvement Amendments (CLIA) of 1988 certified or other similarly certified laboratories. The classification of BRAF fusion included BRAF tandem duplication, BRAF rearrangement and BRAF fusions involving partner genes other than KIAA1549.
Sex and/or gender were not considered in the trial design as no sex differences have been seen in previous clinical trials in pLGG, although, in line with rates generally seen in childhood cancer50, there is one report of the incidence of pLGG being slightly higher in males than in females51. The current trial recruited any patient independent of sex or gender. The sex of the participants was based on either parental report or self-report. The gender of patients was not captured or considered as part of this trial as, at the time the protocol was written and the FIREFLY-1 trial was initiated, there was less of a focus than currently on collecting gender-related information.
Tovorafenib was administered at the recommended phase 2 dose of 420 mg m−2 (not to exceed 600 mg) by mouth (tablet or liquid formulation), once weekly, on days 1, 8, 15 and 22 of 28-d cycles. Treatment cycles were to be repeated every 28 d until radiographic evidence of disease progression as determined by the treating investigator according to RANO-HGG criteria, unacceptable toxicity, decision to enter a drug holiday period, patient withdrawal of consent or death. Patients who had radiographic evidence of disease progression were allowed to continue tovorafenib treatment if, in the opinion of the investigator and approved by the sponsor, the patient was deriving clinical benefit from continuing trial treatment. Patients were to be treated with tovorafenib for a planned period of 26 cycles (approximately 24 months), after which they could continue on tovorafenib or, at any point, opt to enter a drug holiday period. During this drug holiday period, patients could be re-treated with tovorafenib if there was radiographic evidence of disease progression.
An independent data safety monitoring board (DSMB) was established before initiation of the trial and was in place for the duration of the trial. Medical monitoring of the trial was provided by the sponsor and used a safety review committee that included the principal investigator (or their designee) from each active clinical site.
The protocol, protocol amendments, informed consent form, pediatric assent form, investigator brochure and other relevant documents were approved by an institutional review board/independent ethics committee at each trial site. As applicable according to local regulations, the protocol and all protocol amendments were reviewed and approved by each pertinent competent authority.
This trial was conducted in accordance with the protocol and consensus ethical principles derived from international guidelines, including the Declaration of Helsinki, Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines, applicable International Council for Harmonization Good Clinical Practice (GCP) guidelines and other applicable laws and regulations. All patients and/or their legally authorized representative provided written informed consent and pediatric assent before enrollment in the trial, according to local regulations. No direct compensation was provided to patients or families for participating in the trial.
Data collection
Clinical data required by the protocol were entered into the electronic case report forms (eCRFs) and used a fully validated secure web-enabled electronic data capture (EDC) system, Medidata Classic Rave 2022.3.2, which is compliant with 21 CRF Part 11 requirements. Automatic validation edit checks in the EDC system and offline listings were programmed to capture data discrepancies in the eCRFs and allowed modification and validation of the entered data. The investigator verified and signed off the eCRFs in the EDC system to confirm that the clinical data captured were complete and accurate. The sponsor can attest that all data and metadata will be archived in perpetuity. The data are in the EDC system and the trial master file (TMF), which are retained in perpetuity. In addition, these data have been filed with the US FDA.
Eligibility
Full inclusion and exclusion criteria are listed in the trial protocol, located in the Supplementary Information for this publication. In brief, eligible patients in arm 1 were aged 6 months to 25 years, inclusive, with a histopathologically verified pLGG, which had previously been treated with at least one line of prior systemic therapy with evidence of radiographic progression, a documented known activating BRAF alteration and measurable disease as defined by RANO-HGG criteria, a Lansky (aged <16 years) or Karnofsky (aged ≥16 years) performance score of ≥50 and adequate organ function. Radiation therapy to the measurable lesion(s) must have been completed at least 6 months before administration of tovorafenib, and patients must have fully recovered from the acute toxic effects of all prior anti-cancer chemotherapy and from any prior surgery. Patients must have had adequate bone marrow and organ function, including a left ventricular ejection fraction (LVEF) of ≥50% as measured by ECG or multiple-gated acquisition (MUGA) scan or fractional shortening (FS) ≥25% as measured by ECG52, within 28 d before the first dose of tovorafenib. Tumor tissue (archival) was obtained at enrollment whenever available. Tissue biopsy was required during screening only if an archival tumor tissue sample was not available.
Patients were excluded if their tumor harbored an additional previously known or expected to be activating molecular alteration; if they had symptoms of clinical progression without radiographically recurrent or radiographically progressive disease; if they had a known or suspected diagnosis of neurofibromatosis type 1 by genetic testing or current diagnostic criteria; if they had a history or current evidence of central serous retinopathy, retinal vein occlusion or ophthalmopathy present at baseline that would be considered a risk factor for either; if they had clinically significant active cardiovascular disease; or if they were neurologically unstable despite adequate treatment.
Trial endpoints
The assessment of response was undertaken using three different radiological response assessment criteria: RANO-HGG criteria, which assess tumor response primarily based on T1-weighted, contrast-enhanced imaging, and RAPNO-LGG and RANO-LGG criteria, both of which assess tumor response based primarily on non-enhancing disease by T2/FLAIR. The characteristics of these different response criteria are summarized in Extended Data Table 1. As per the trial design, patients were initially enrolled based on investigator assessment of eligibility per RANO-HGG criteria. Investigator response assessments per RANO-HGG were also the criteria on which cessation of treatment due to PD were based. Response was subsequently analyzed per all three criteria by blinded independent central review.
The primary endpoint in arm 1 was ORR, defined as the proportion of patients with a confirmed response of CR or PR according to RANO-HGG criteria, as assessed by an IRC. Secondary endpoints for arm 1 included CBR, PFS, DOR and TTR, as assessed by the IRC using RANO-HGG criteria. CBR is defined as the proportion of patients with a confirmed response or SD lasting any length of time, 6 months or more or 12 months or more. ORR, CBR, PFS, DOR and TTR were also assessed by the IRC according to RAPNO criteria. Secondary endpoints for safety included evaluation of AEs, laboratory abnormalities and cardiac function assessments (change from baseline in QTcF, PR interval, QRS interval, heart rate or ECG waveform morphology). Post hoc exploratory endpoints for arm 1 included ORR and CBR according to RANO-LGG criteria by IRC assessment. For RAPNO and RANO-LGG ORR assessments, patients with confirmed MRs were considered responders in accordance with published guidelines. Changes in quality of life and health utilities measures were exploratory objectives.
Assessments
Disease assessments in arm 1 were conducted by MRI of the brain and spine and were performed at screening up to 28 d before first dose, at the end of cycle 3 and then at the end of every three cycles thereafter. Spinal scans were only required to be repeated after screening in patients with known or clinically suspected intraspinal disease. Patients who had an optic pathway glioma (OPG) or underlying visual function deficit related to the primary malignancy had a visual acuity examination every time they had a radiographic disease assessment.
A central imaging laboratory was used. Imaging Endpoints (IE) is a research and imaging core laboratory providing blinded independent central review of response assessments with dual reader plus adjudication paradigm using neuro-radiologists trained in all three response assessment criteria as readers for the following assessments: RANO-HGG criteria, RAPNO-LGG criteria and RANO-LGG criteria. All activities at IE meet or exceed GCP standards, and IE underwent a GCP audit by the sponsor. A prospectively designed imaging charter was developed for the FIREFLY-1 study before the initiation of the study. This outlined the processes for initial imaging review, data transfers and data review, and queries were followed throughout the study. IE functions as the centralized imaging core laboratory responsible for the collection, quality control, archiving and blinded independent central review (BICR) of imaging for the FIREFLY-1 trial. IE is responsible for management of the image analysis system, reporting methods, implementation of the analysis criteria and reader management, including qualification, training and oversight.
Reader performance was assessed by evaluating reader variability at defined and prespecified milestones during ongoing imaging interpretation. Variability metrics included inter-reader and intra-reader variability to monitor for consistency of reads. If the reader acceptance rate fell outside the caution or alert limits, IE determined the appropriate unbiased action(s).
For this report, safety was assessed in the arm 1 and 2 safety analysis set, which comprised all enrolled patients who received at least one dose of trial treatment. The assessment period for AEs was from the first dose of tovorafenib until 30 d after the last dose. For the current trial, an AE was defined as treatment emergent if it occurred at any time after the first dose of trial drug until 30 d after the last dose of trial drug. A TRAE was any treatment-emergent event that the investigator assessed had at least a reasonable possibility of having a causal relationship with the trial drug based on temporal association with initiation of treatment and assessment of other potential etiologies. An AE was considered serious if it met one of the following criteria: required or prolonged hospitalization, was life-threatening, caused disability or was considered a medically important event by the investigator (regardless of symptoms or Common Terminology Criteria for Adverse Events (CTCAE) grade).
Routine laboratory tests were performed locally and included pregnancy tests for female patients of childbearing potential (at screening and on day 1 of every cycle); assessments of hematology parameters and serum chemistries (at screening, days 1 and 15 of cycle 1, day 1 of cycle 2 and every cycle thereafter); and thyroid function (at screening, on day 1 of cycles 1–3 and every other cycle thereafter). Scheduled cardiac function assessments included independently centrally reviewed 12-lead resting ECGs (performed in triplicate at baseline, on days 1 and 15 of cycle 1, on day 1 of cycles 2 and 4 and on day 1 of every third cycle thereafter) and ECGs or MUGA scans (conducted throughout by the same technique, on day 1 of cycles 2 and 4 and on day 1 of every third cycle thereafter). CPK level was assessed at screening, on day 1 of cycles 2 and 4 and on day 1 of every fourth cycle thereafter.
In patients 2 years of age or older, health-related quality of life was assessed using the PedsQL-Core, PedsQL-Cancer and PROMIS assessments for the patient or their parent/caregiver every third cycle. The PROMIS questionnaire was administered only to English-speaking patients enrolled in the United States, Australia and the United Kingdom.
Statistical considerations
In terms of the ‘evaluable’ population, patients ‘evaluable for efficacy’ were all patients enrolled in the trial who received at least one dose of trial treatment and met the definition for the prespecified efficacy analyses criteria (RANO-HGG, RAPNO and RANO-LGG)30,31,32; those in the ‘evaluable for DOR’ population were patients evaluable for efficacy who had a best overall confirmed response of CR, PR or MR (for RAPNO and RANO-LGG criteria). The ‘safety’ population consisted of all patients enrolled in the trial who received at least one dose of trial treatment. This report presents efficacy data from the evaluable population in arm 1 and the safety data from arms 1 and 2 as of a 5 June 2023 data cutoff.
The primary endpoint analysis was performed in the arm 1 evaluable population, which included all enrolled patients who received at least one dose of tovorafenib and had measurable disease at baseline per RANO-HGG criteria as determined by the IRC. A sample size of 60 patients in the evaluable population was considered to provide 88% power to reject the null hypothesis ORR of 21%, at the two-sided 0.05 level, assuming that the true underlying ORR of tovorafenib was 40% in this disease population. An exact binomial test was used for hypothesis testing. Responses per RAPNO and RANO-LGG criteria were evaluated in evaluable populations, which included all enrolled patients who received at least one dose of tovorafenib and had measurable disease at baseline per RAPNO and RANO-LGG criteria, respectively, as determined by the IRC.
Prespecified subgroup analysis of the uniformity of the treatment effects for ORR in arm 1 was planned for subgroups defined by BRAF alteration (BRAF fusion versus BRAF mutation), number of prior lines of therapies, prior MAPKi status (prior MEKi and/or prior BRAFi), sex, age group (6 months to <2 years of age, 2 years to <6 years of age, 6 years to <12 years of age, 12 years to <16 years of age and 16 years to ≤25 years of age) and race.
The ORR and CBR were calculated with 95% CIs determined using the Clopper–Pearson method. PFS and DOR were estimated by the Kaplan–Meier method and were summarized along with the corresponding two-sided 95% CI. Waterfall plots were generated for each patient’s best percentage change in sum of perpendicular diameters of measurable lesions.
Safety endpoints were analyzed using descriptive statistics based on the safety population in arms 1 and 2. AEs were coded by system organ class and preferred terms using the Medical Dictionary for Regulatory Activities (MedDRA) version 23.1 and graded according to the National Cancer Institute CTCAE version 5.0.
Statistical analyses were carried out using SAS version 9.4.
Management of cutaneous AEs
Guidance for the management of rash/dermatitis was included in the trial protocol, in line with the stepwise approach proposed by Song et al.53 for the prevention and treatment of common cutaneous adverse reactions to BRAF, MEK and mTOR inhibitors in children with CNS tumors53. Dermatology assessment was performed at baseline and was symptom directed thereafter. Patients were to be referred to a dermatology department if cutaneous symptoms were impairing function (for example, if the patient could not sleep or sit still) or were psychosocially bothersome and/or when management techniques failed to resolve the condition. It was recommended that all trial patients followed a gentle skin care routine comprising short lukewarm showers/baths, the use of unscented, gentle cleansers and the application of unscented thick moisturizers (creams over lotions) immediately after showers. In addition, the prophylactic use of SPF 30+ sunscreen whenever going outside, with this reapplied every 2 h, and the wearing of sun-protective clothing were recommended for all patients. Specific guidance was also provided to manage mild or moderate/severe follicular, eczematous, paronychia or periungual reactions or hand–foot syndrome.
In the event of grade 2 macular or papular eruption, erythema with pruritus or other associated symptoms, localized desquamation or other lesions covering less than 50% of body surface area (BSA), tovorafenib dose reduction by one dose level once weekly could be considered. In the event of grade 3 or higher severe, generalized erythroderma or macular, papular or vesicular eruption, desquamation covering ≥50% of BSA or generalized exfoliative, ulcerative or bullous dermatitis, tovorafenib dosing was to be delayed until the condition improved, a dermatologist was consulted and a dose reduction by two dose levels or holding tovorafenib administration until resolution to grade 1 or baseline could be considered.
Key protocol amendments
Current version 3.0, 21 October 2021
The protocol was amended primarily to add two new arms to the trial, to add a powder for reconstitution formulation of tovorafenib and to incorporate other changes based on feedback from regulatory authorities. This version of the full trial protocol (some confidential information redacted) is in the Supplementary Information supporting the article.
Version 2.0, 23 October 2020
The protocol was amended primarily to change the recommended phase 2 dose of tovorafenib from 530 mg m−2 to 420 mg m−2 and decrease the maximum dose from 800 mg to 600 mg once weekly. The planned number of cycles was reduced from 27 cycles to 26 cycles (patients could continue on trial treatment beyond this if criteria were met), and the upper age limit was increased from 18 years to 25 years, inclusive.
Clinical trial registration
The study is registered on ClinicalTrials.gov as NCT04775485 and on EudraCT as 2020-003657-30.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The trial protocol (confidential information redacted) is provided in the Supplementary Information. The authors declare that all data supporting the findings of this trial are available within the article and the Supplementary Information. Requests for full datasets will be considered after completion of the trial and analysis of the data, which is anticipated to be in December 2024. To request individual participant data associated with any Day One Biopharmaceuticals clinical trial, email clinical@dayonebio.com. All requests will be evaluated within 8 weeks.
References
-
Ryall, S., Tabori, U. & Hawkins, C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol. Commun. 8, 30 (2020).
-
Gnekow, A. K. et al. SIOP-E-BTG and GPOH guidelines for diagnosis and treatment of children and adolescents with low grade glioma. Klin. Padiatr. 231, 107–135 (2019).
-
Moreira, D. C. et al. Tackling pediatric low-grade gliomas: a global perspective. JCO Glob. Oncol. 9, e2300017 (2023).
-
Ater, J. L. et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J. Clin. Oncol. 30, 2641–2647 (2012).
-
Heitzer, A. M. et al. Neuropsychological outcomes of patients with low-grade glioma diagnosed during the first year of life. J. Neurooncol. 141, 413–420 (2019).
-
Jones, D. T. W. et al. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 20, 160–173 (2018).
-
Morrison, D. K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 4, a011254 (2012).
-
Johnson, A. et al. Comprehensive genomic profiling of 282 pediatric low- and high-grade gliomas reveals genomic drivers, tumor mutational burden, and hypermutation signatures. Oncologist 22, 1478–1490 (2017).
-
Ryall, S. et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell 37, 569–583 (2020).
-
Behling, F. & Schittenhelm, J. Oncogenic BRAF alterations and their role in brain tumors. Cancers (Basel) 11, 794 (2019).
-
Collins, V. P., Jones, D. T. & Giannini, C. Pilocytic astrocytoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 129, 775–788 (2015).
-
Faulkner, C. et al. BRAF fusion analysis in pilocytic astrocytomas: KIAA1549-BRAF 15-9 fusions are more frequent in the midline than within the cerebellum. J. Neuropathol. Exp. Neurol. 74, 867–872 (2015).
-
Bouffet, E. et al. Dabrafenib plus trametinib in pediatric glioma with BRAF V600 mutations. N. Engl. J. Med. 389, 1108–1120 (2023).
-
Dabrafenib prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/202806s025lbl.pdf
-
Bouffet, E. et al. Efficacy and safety of trametinib monotherapy or in combination with dabrafenib in pediatric BRAFV600-mutant low-grade glioma. J. Clin. Oncol. 41, 664–674 (2023).
-
Hargrave, D. R. et al. Efficacy and safety of dabrafenib in pediatric patients with BRAF V600 mutation-positive relapsed or refractory low-grade glioma: results from a phase I/IIa study. Clin. Cancer Res. 25, 7303–7311 (2019).
-
Yaeger, R. & Corcoran, R. B. Targeting alterations in the RAF-MEK pathway. Cancer Discov. 9, 329–341 (2019).
-
Botton, T. et al. Genetic heterogeneity of BRAF fusion kinases in melanoma affects drug responses. Cell Rep. 29, 573–588 e577 (2019).
-
Karajannis, M. A. et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol. 16, 1408–1416 (2014).
-
Fangusaro, J. et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 20, 1011–1022 (2019).
-
Perreault, S. et al. A phase 2 study of trametinib for patients with pediatric glioma with activation of the MAPK/ERK pathway. Neuro Oncol. 23, vi59–vi60 (2021).
-
Robison, N. et al. MEK162 (binimetinib) in children with progressive or recurrent low-grade glioma: a multi-institutional phase II and target validation study. Neuro Oncol. 24, i191–i192 (2022).
-
Trippett, T. et al. Cobimetinib in pediatric and young adult patients with relapsed or refractory solid tumors (iMATRIX-cobi): a multicenter, phase I/II study. Target. Oncol. 17, 283–293 (2022).
-
Manoharan, N., Liu, K. X., Mueller, S., Haas-Kogan, D. A. & Bandopadhayay, P. Pediatric low-grade glioma: targeted therapeutics and clinical trials in the molecular era. Neoplasia 36, 100857 (2023).
-
Sun, Y. et al. A brain-penetrant RAF dimer antagonist for the noncanonical BRAF oncoprotein of pediatric low-grade astrocytomas. Neuro Oncol. 19, 774–785 (2017).
-
Rasco, D. W. et al. Phase 1 study of the pan-RAF inhibitor tovorafenib in patients with advanced solid tumors followed by dose expansion in patients with metastatic melanoma. Cancer Chemother. Pharmacol. 92, 15–28 (2023).
-
Lee, J. et al. Clinical activity of the type II pan-RAF inhibitor tovorafenib in BRAF-fusion melanoma. Poster 174 presented at the 19th European Association of Dermato-Oncology Congress. https://publications.dayonebio.com/Clinical+activity+of+the+type+II+pan-RAF+inhibitor+tovorafenib+in+BRAF-fusion+melanoma.pdf (2023).
-
Offer, K. et al. Activity of type II RAF inhibitor tovorafenib in a pediatric patient with a recurrent spindle cell sarcoma harboring a novel SNX8–BRAF gene fusion. JCO Precis. Oncol. 7, e2300065 (2023).
-
Wright, K. et al. PNOC014: phase IB study results of DAY101(tovorafenib) for children with low-grade gliomas (LGGs) and other RAS/RAF/MEK/ERK pathway-activated tumors. Neuro Oncol. 24, vii84 (2022).
-
Wen, P. Y. et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 28, 1963–1972 (2010).
-
Fangusaro, J. et al. Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 21, e305–e316 (2020).
-
Wen, P. Y. et al. Response Assessment in Neuro-Oncology clinical trials. J. Clin. Oncol. 35, 2439–2449 (2017).
-
Bouffet, E. et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J. Clin. Oncol. 30, 1358–1363 (2012).
-
Kilburn, L. et al. Clinical activity of pan-RAF inhibitor tovorafenib in the registrational pediatric low-grade glioma arm of the phase 2 FIREFLY-1 (PNOC026) study. J. Clin. Oncol. 41, 10004 (2023).
-
Fangusaro, J. et al. A phase II trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: a Pediatric Brain Tumor Consortium study. Neuro Oncol. 23, 1777–1788 (2021).
-
Banerjee, A. et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol. 19, 1135–1144 (2017).
-
Ma, Y., Wang, Q., Dong, Q., Zhan, L. & Zhang, J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am. J. Cancer Res. 9, 1546–1553 (2019).
-
Monch, S. et al. Patterns of pseudoprogression across different cancer entities treated with immune checkpoint inhibitors. Cancer Imaging 23, 58 (2023).
-
Borcoman, E. et al. Novel patterns of response under immunotherapy. Ann. Oncol. 30, 385–396 (2019).
-
Reitman, Z. J. et al. Mitogenic and progenitor gene programmes in single pilocytic astrocytoma cells. Nat. Commun. 10, 3731 (2019).
-
Selt, F. et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J. Neurooncol. 149, 499–510 (2020).
-
Gross, A. M. et al. Selumetinib in children with inoperable plexiform neurofibromas. N. Engl. J. Med. 382, 1430–1442 (2020).
-
Mincu, R. I. et al. Cardiovascular adverse events associated with BRAF and MEK inhibitors: a systematic review and meta-analysis. JAMA Netw. Open 2, e198890 (2019).
-
Stjepanovic, N., Velazquez-Martin, J. P. & Bedard, P. L. Ocular toxicities of MEK inhibitors and other targeted therapies. Ann. Oncol. 27, 998–1005 (2016).
-
Donofrio, C. A. et al. Pediatric cerebellar pilocytic astrocytoma presenting with spontaneous intratumoral hemorrhage. Neurosurg. Rev. 43, 9–16 (2020).
-
Ishi, Y. et al. Association of the FGFR1 mutation with spontaneous hemorrhage in low-grade gliomas in pediatric and young adult patients. J. Neurosurg. 134, 733–741 (2020).
-
Matsuura, C. et al. [Diffuse astrocytoma with pilomyxoid features presenting as intratumoral hemorrhage: a case report]. No Shinkei Geka 46, 1073–1079 (2018).
-
Ramdurg, S. R. & Maitra, J. A rare case of infantile cerebellar pilocytic astrocytoma and thrombocytopenia presenting with intratumoral hemorrhage. J. Pediatr. Neurosci. 11, 249–251 (2016).
-
Collet-Solberg, P. F. et al. Endocrine outcome in long-term survivors of low-grade hypothalamic/chiasmatic glioma. Clin. Endocrinol. (Oxf.) 47, 79–85 (1997).
-
Steliarova-Foucher, E. et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. 18, 719–731 (2017).
-
Gnekow, A. K. et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 14, 1265–1284 (2012).
-
Tissot, C., Singh, Y. & Sekarski, N. Echocardiographic evaluation of ventricular function—for the neonatologist and pediatric intensivist. Front. Pediatr. 6, 79 (2018).
-
Song, H. et al. Cutaneous reactions to targeted therapies in children with CNS tumors: a cross-sectional study. Pediatr. Blood Cancer 66, e27682 (2019).
Acknowledgements
This trial was funded by Day One Biopharmaceuticals. We thank the patients, families, caregivers and clinical investigators for their participation in the FIREFLY-1 trial. We are deeply grateful for the site coordinators and trial staff who are instrumental in making this work possible. The authors would like to thank the Pacific Pediatric Neuro-Oncology Consortium (PNOC) for their partnership in the conduct of this trial. We also would like to acknowledge and thank the PNOC014 investigators and trial team (principal investigator: K. Wright) for generating the initial observation of the activity of tovorafenib in pLGG in their phase 1 trial. We also thank M. C. Cox for his contribution to protocol development and trial management. Medical writing support was provided by M. Hoke and S. Govinda Raju of Day One Biopharmaceuticals and J. Heighway and A. Cleasby of Cancer Communications and Consultancy Ltd., funded by Day One Biopharmaceuticals. D.S.Z. is supported by grants from the National Health and Medical Research Council (Synergy Grant 2019056 and Leadership Grant APP2017898) and Cancer Institute New South Wales Program Grant TPG2037. D.H. is supported by funding from the National Institute for Health and Care Research (NIHR) Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the National Health Service, the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
All authors had access to primary clinical trial data. All authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. All authors contributed substantially to the concept and design of the manuscript, data analysis and interpretation and writing of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.B.K. has received consulting fees from Blueprint Medicine as DSMB Chair and has contracted institutional research with Novartis, Regeneron Pharmaceuticals, Day One Biopharmaceuticals, Spring Works Therapeutics, Bristol Myers Squibb and SonALAsense. L.B.K. also owns stock in Onconova Therapeutics. J.R.H. has received honoraria for consultation from Bayer, Alexion Pharma and Boxer Capital. P.H.D. is on an advisory board with Alexion and is part of the Alexion ICI Sprinkle Study. S.P. is on advisory boards with Bayer, Alexion and Esai and has received research support from Novartis, Bayer and Roche. D.S.Z. has received consulting/advisory board fees from Bayer, AstraZeneca, Accendatech, Novartis, Day One Biopharmaceuticals, FivePhusion, Amgen, Alexion and Norgine and has received research support from Accendatech. O.W. is on advisory boards with Novartis, Janssen, Roche, Bristol Myers Squibb and AstraZeneca and has received research grants from Day One Biopharmaceuticals, Biomed Valley Discovery, Bristol Myers Squibb, Syndax and PreComb. H.J.K. is on advisory boards with Novartis, Jazz Pharmaceuticals, Takeda, Cartexell and GPCR. D.H. is on advisory boards with Alexion/AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Day One Biopharmaceuticals, Janssen, Novartis and Roche and has received research grants from Alexion/AstraZeneca and Roche. V.L. is on an advisory board with Alexion. C.K. is a study chair of an investigator-sponsored trial for which Day One Biopharmaceuticals provides drug and research support; she also has research relationships with other industry partners. D.S. is on an advisory board with Alexion. L.M., X.Z., A.W., D.D., P.M., I.C. and S.C.B. are employees of Day One Biopharmaceuticals and have received Day One Biopharmaceuticals stock and stock options. K.N. has received advisory board or consulting fees from Y-mAbs, EUSA Pharma, Bayer and Eli Lilly. All remaining authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Sonia Partap and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Inclusion and ethics statement All collaborators of this study who have fulfilled the criteria for authorship required by Nature Portfolio journals have been included as authors, as their participation was essential for the design and implementation of the study. Roles and responsibilities were agreed among collaborators ahead of the research. This work includes findings that are locally relevant, which have been determined in collaboration with local partners. This research was not severely restricted or prohibited in the setting of the researchers and does not result in stigmatization, incrimination, discrimination or personal risk to participants. Local and regional research relevant to our study was taken into account in citations.
Extended data
Extended Data Fig. 1 Tumor kinetics.
Fig. 1a shows tumor kinetics in patients who progressed while on therapy per RANO-HGG but continued to receive tovorafenib and who had at least one assessment from a scheduled visit post-PD. Fig. 1b,c show tumor kinetics in patients with best response of progressive disease according to RAPNO and RANO-LGG criteria, respectively. In some patients, who continued therapy due to the absence of a progressive disease assessment per RANO-HGG criteria, an apparent initial increase in tumor size per RAPNO and RANO-LGG criteria was subsequently followed by a sustained decrease in size, suggesting that the initial apparent increase may not represent true progression. HGG, high-grade glioma; LGG, low-grade glioma; PD, progressive disease; RANO, Response Assessment in Neuro-Oncology; RAPNO, Response Assessment in Pediatric Neuro-Oncology.
Extended Data Fig. 2 Swimlane plot of time to response and duration of therapy per RANO-LGG criteria.
In patients with response, symbols indicate the start of response (MR or PR). If initial response improved with continued treatment (from MR to confirmed PR), both the timepoint of the initial response and the timepoint that response initially improved are marked accordingly. BRAFi, BRAF inhibitor; LGG, low-grade glioma; MEKi, MEK inhibitor; MR, minor response; PR, partial response; RANO, Response Assessment in Neuro-Oncology.
Extended Data Fig. 3 Forest plot of response according to RAPNO in subgroups defined by baseline characteristics.
Filled circles represent the overall response rates, and whiskers represent the 95% confidence intervals. Other races included Asian (n = 5), Black (n = 2), Multiple (n = 3), and Other (n = 6). There were no Native Hawaiian or other Pacific Islander or American Indian or Alaska Native participants. No race information was missing. Ex, External to; US, United States.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kilburn, L.B., Khuong-Quang, DA., Hansford, J.R. et al. The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: the phase 2 FIREFLY-1 trial. Nat Med (2023). https://ift.tt/MaWleLm
-
Received:
-
Accepted:
-
Published:
-
DOI: https://ift.tt/MaWleLm
"type" - Google News
November 17, 2023 at 11:34PM
https://ift.tt/tjdy0eQ
The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: the phase 2 FIREFLY-1 trial - Nature.com
"type" - Google News
https://ift.tt/6HyK0wJ
https://ift.tt/S6bNz7l
Bagikan Berita Ini














0 Response to "The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: the phase 2 FIREFLY-1 trial - Nature.com"
Post a Comment